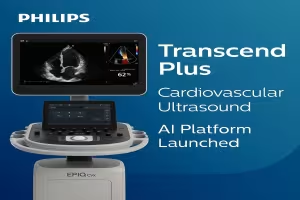
Philips Expands Cardiovascular Ultrasound Leadership with Transcend Plus AI Platform
SHERIDAN, WYOMING – August 27, 2025 – Royal Philips has unveiled Transcend Plus, the next-generation upgrade to its EPIQ CVx and Affiniti CVx cardiovascular ultrasound platforms, featuring FDA-cleared artificial intelligence (AI) tools and imaging enhancements that promise to accelerate cardiac workflows and boost diagnostic precision across care settings.
Breakthrough Imaging Enhancements Backed by FDA Clearance